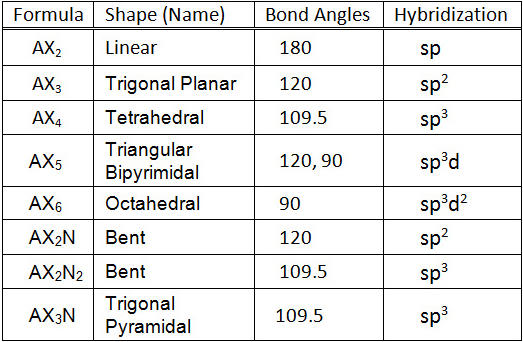
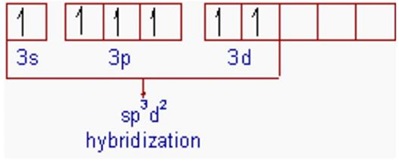
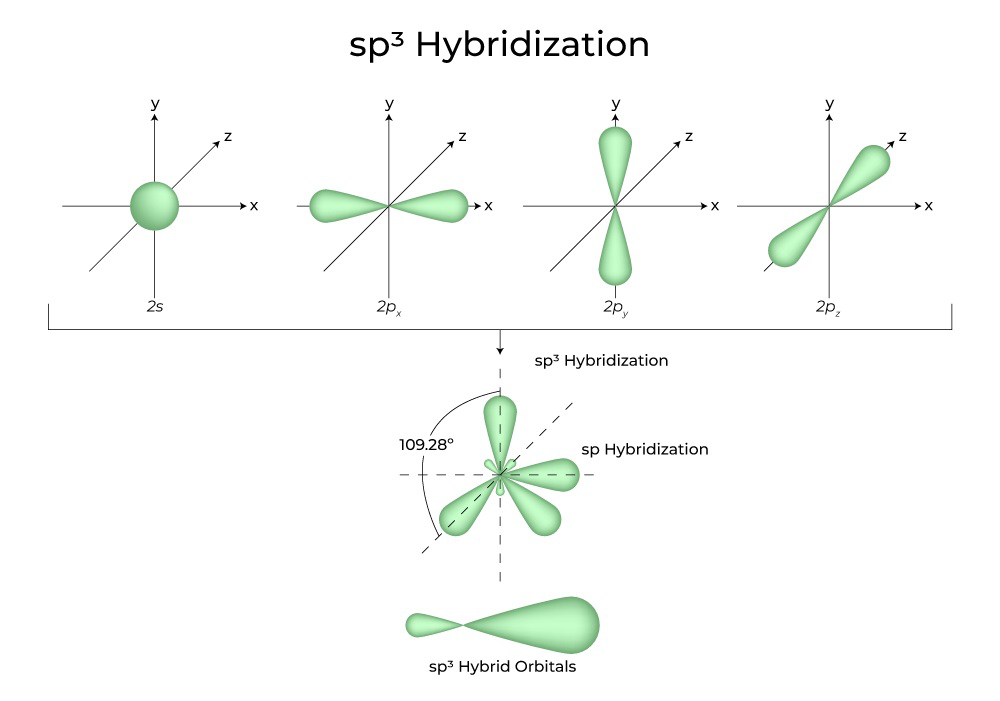
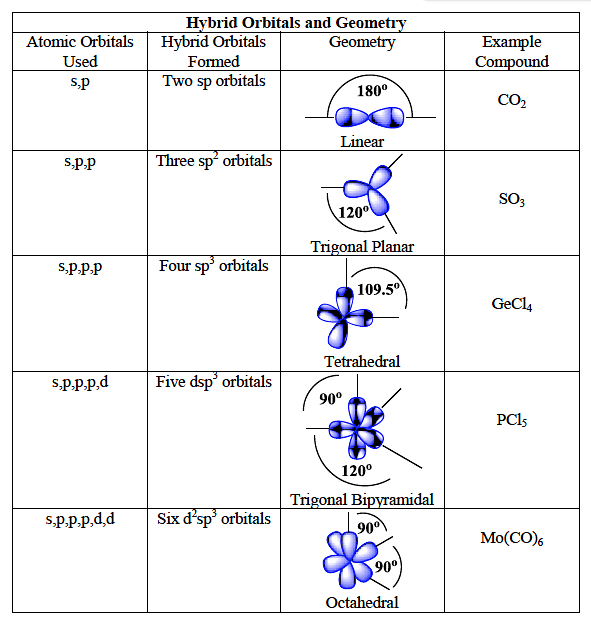
What are the shapes and bond angles of sp, sp2, sp3, sp3d, sp3d2 hybridised orbitals respectively? - Quora

Hybridization || sp3d || sp3d2 || sp3d3 || Formation of PF5, SF6 and IF7 || Chemical Bonding 11th - YouTube
![Which complex compound possesses sp^{3}d^{2} hybridisation?[Fe(NH_{3})_{6}]^{3+}[Fe(CN)_{6}]^{4-}[Fe(CN)_{6}]^{3-}[Fe(Cl)_{6}]^{3-} Which complex compound possesses sp^{3}d^{2} hybridisation?[Fe(NH_{3})_{6}]^{3+}[Fe(CN)_{6}]^{4-}[Fe(CN)_{6}]^{3-}[Fe(Cl)_{6}]^{3-}](https://search-static.byjusweb.com/question-images/toppr_ext/questions/713178_678375_ans_71569d13566849afa3eff10ad47d91bb.png)
Which complex compound possesses sp^{3}d^{2} hybridisation?[Fe(NH_{3})_{6}]^{3+}[Fe(CN)_{6}]^{4-}[Fe(CN)_{6}]^{3-}[Fe(Cl)_{6}]^{3-}

Odes it make any difference if we write hybridisation as sp3d2 or d2sp3 Shape of molecules/ ions Square planar - Chemistry - Chemical Bonding and Molecular Structure - 13299455 | Meritnation.com
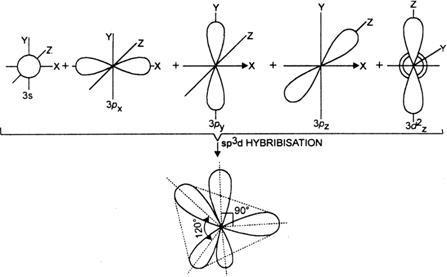
State and explain the geometric arrangements possible in sp3d and sp3d2 hybridisation. Name the d-orbitals involved in these. - Zigya

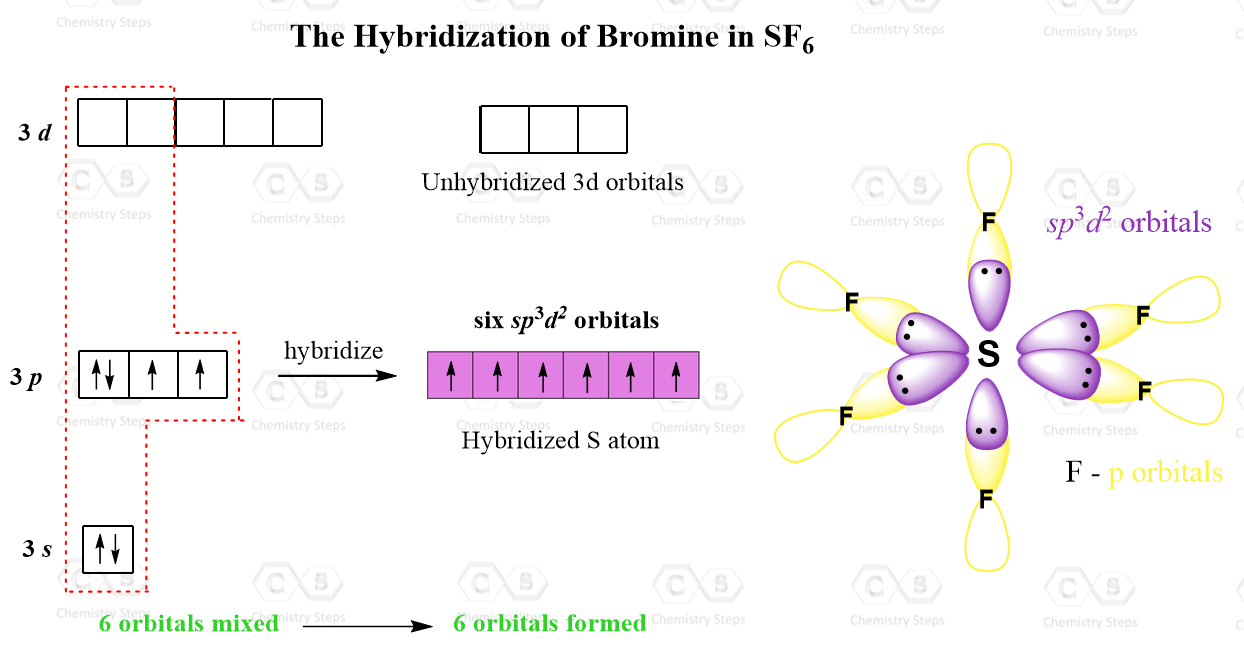
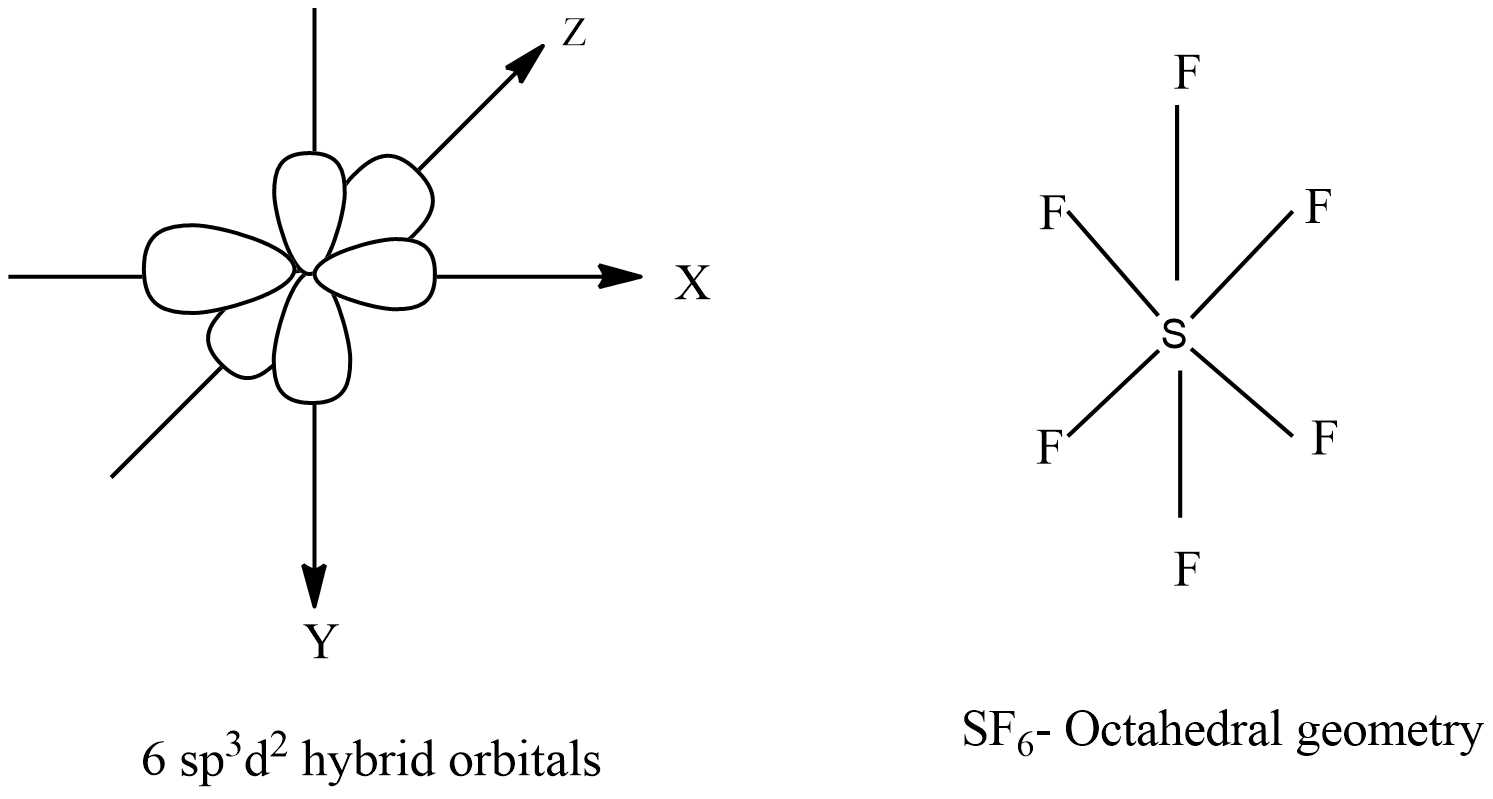

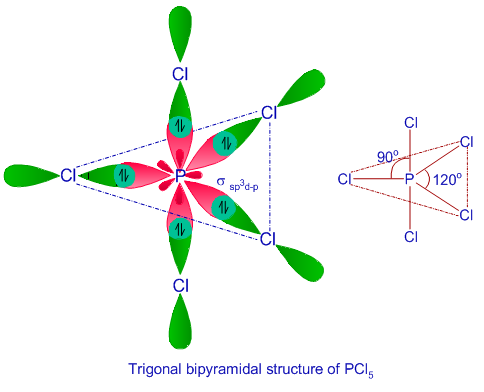

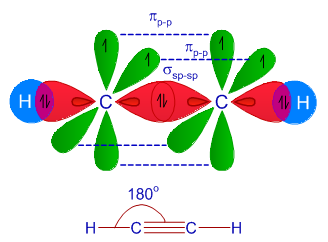
![Solved] Molecule obtained by sp3d2 hybridization has bond angle (s) Solved] Molecule obtained by sp3d2 hybridization has bond angle (s)](https://storage.googleapis.com/tb-img/production/21/08/Reported_23-Aug-2021_Shashi_D8.png)