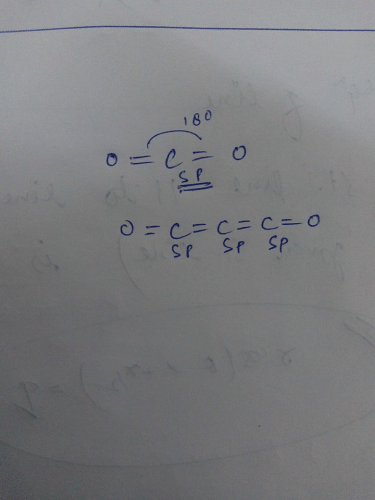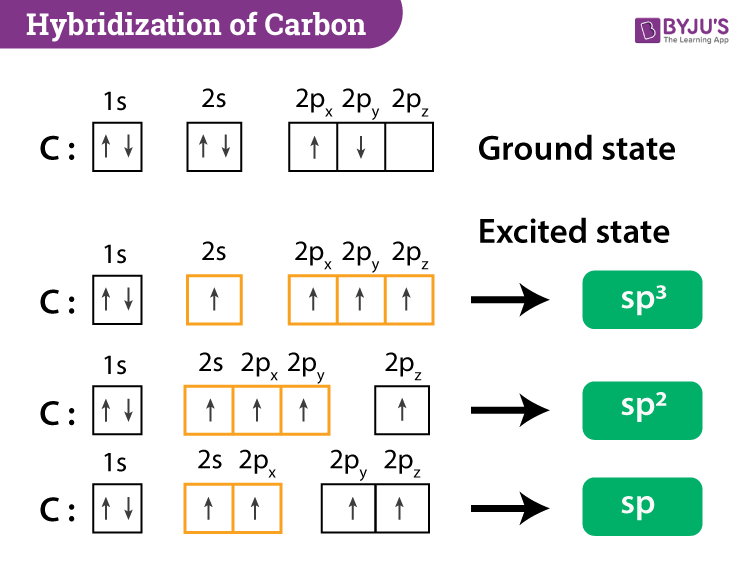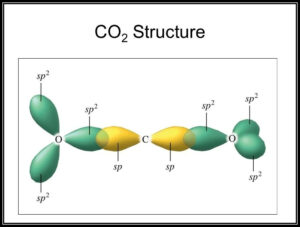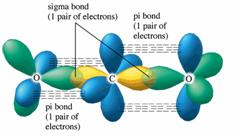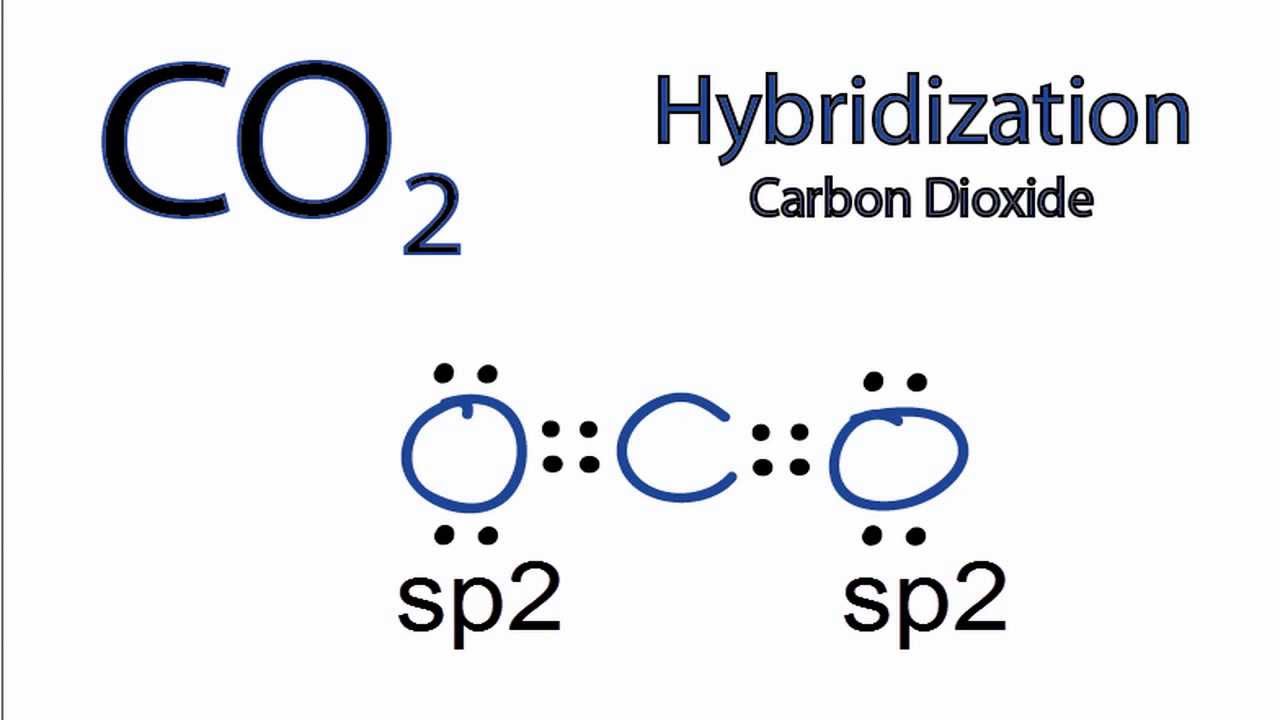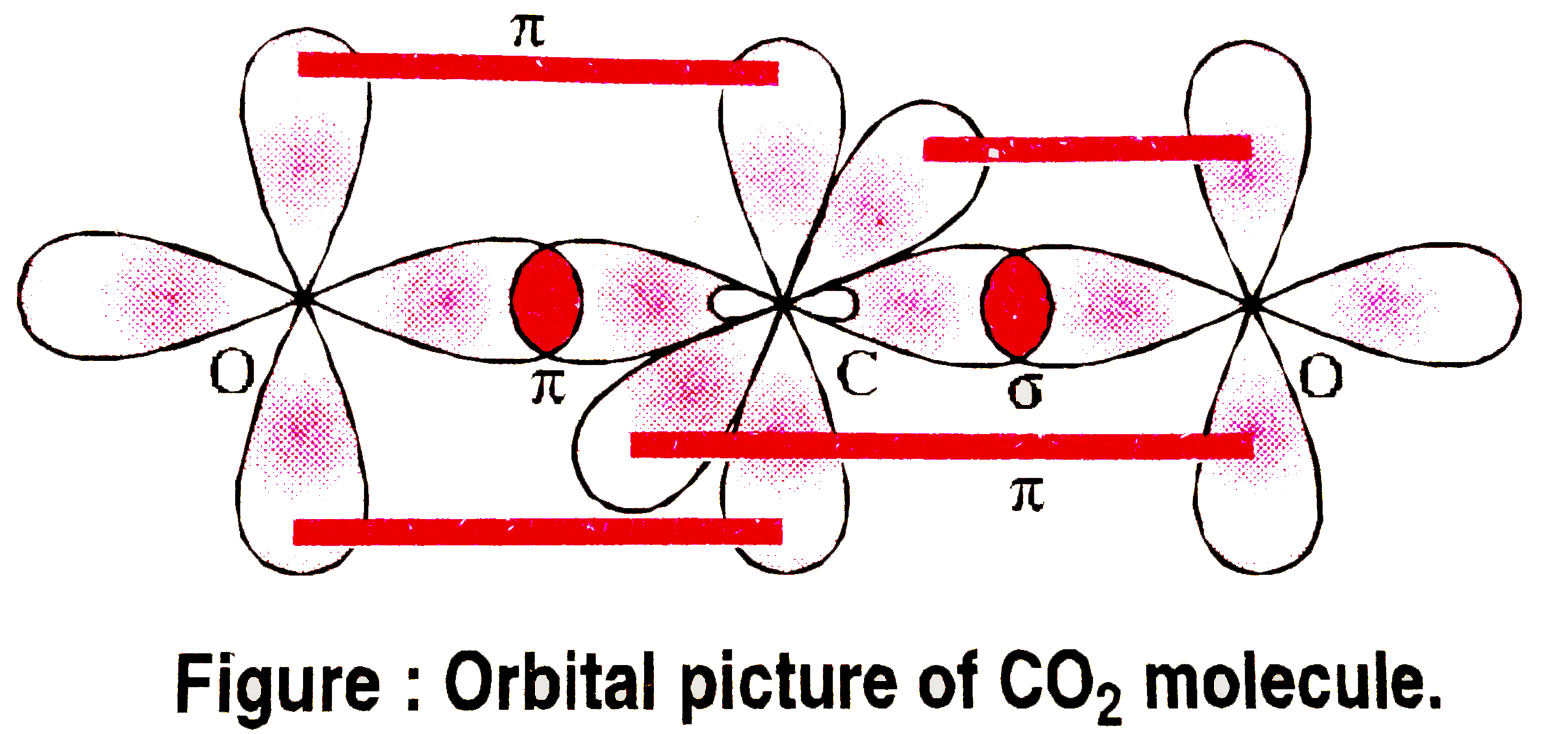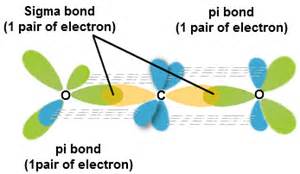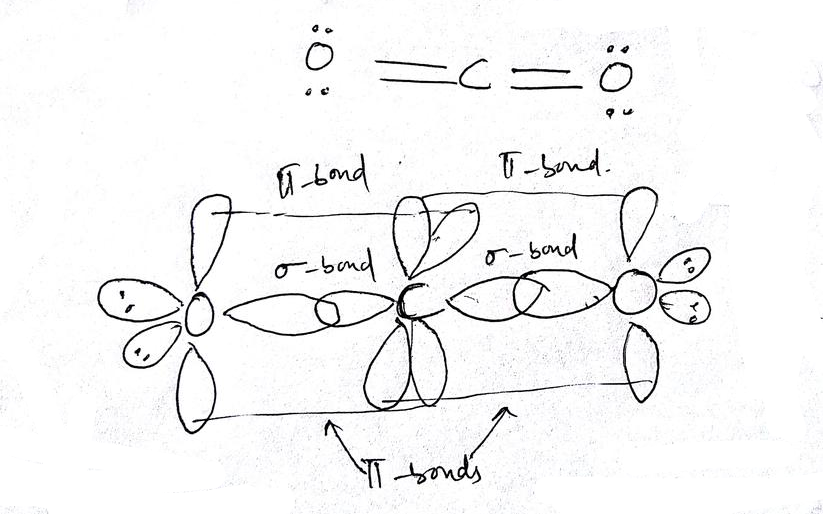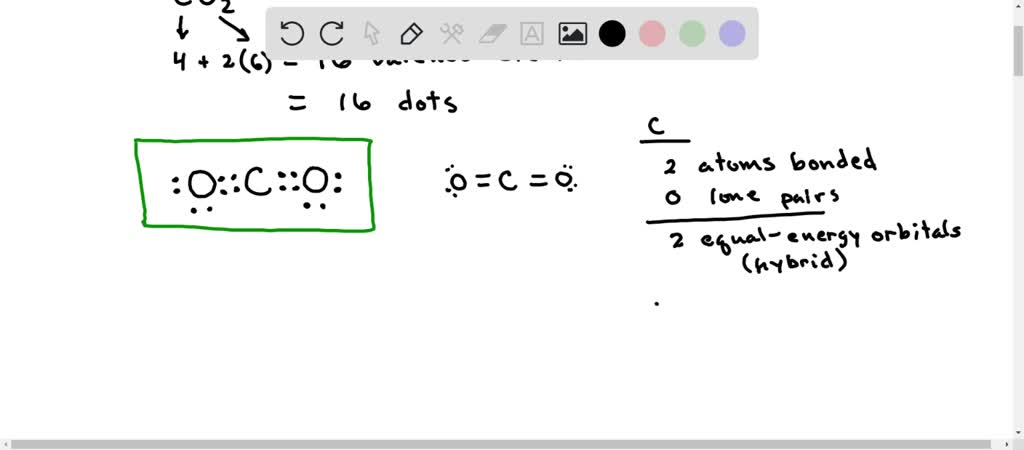
SOLVED: Draw a Lewis structure for CO2. Determine the hybridization on the carbon atom. Which orbitals of the carbon atom remain remain unhybridized? How many sigma and pi bonds are formed?

Draw a Lewis structure and an orbital picture for carbon dioxide, CO2. What kind of hybridization does the carbon atom have? What is the relationship between CO2 and allene? | Homework.Study.com

The molecule CO2 has two C-O double bonds. Describe the bonding in the CO2 molecule. Which involves hybrid - brainly.com

p–d Orbital Hybridization Induced by p-Block Metal-Doped Cu Promotes the Formation of C2+ Products in Ampere-Level CO2 Electroreduction | Journal of the American Chemical Society

p–d Orbital Hybridization Induced by p-Block Metal-Doped Cu Promotes the Formation of C2+ Products in Ampere-Level CO2 Electroreduction | Journal of the American Chemical Society

Allene is structurally related to carbon dioxide, CO2. Draw a picture showing the orbitals involved in the sigma and pi bonds of CO2, and identify the likely hybridization of carbon. | Homework.Study.com
✓ Solved: Describe the bonding on the carbon atom in carbon dioxide, CO2, using valence bond theory.