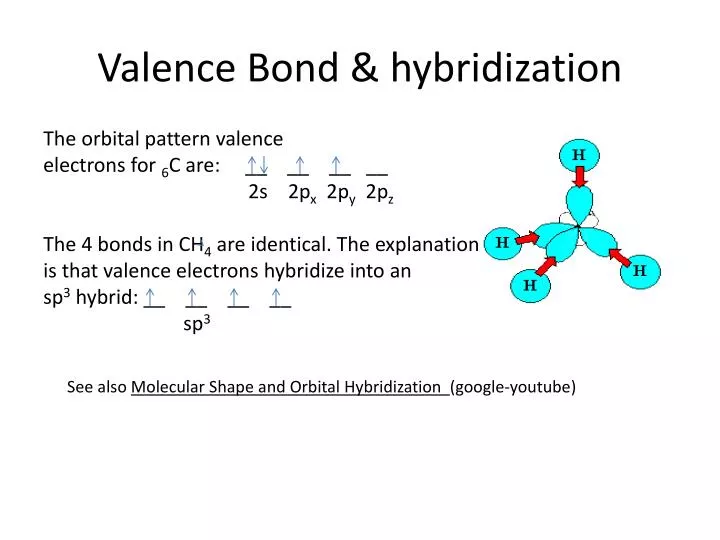
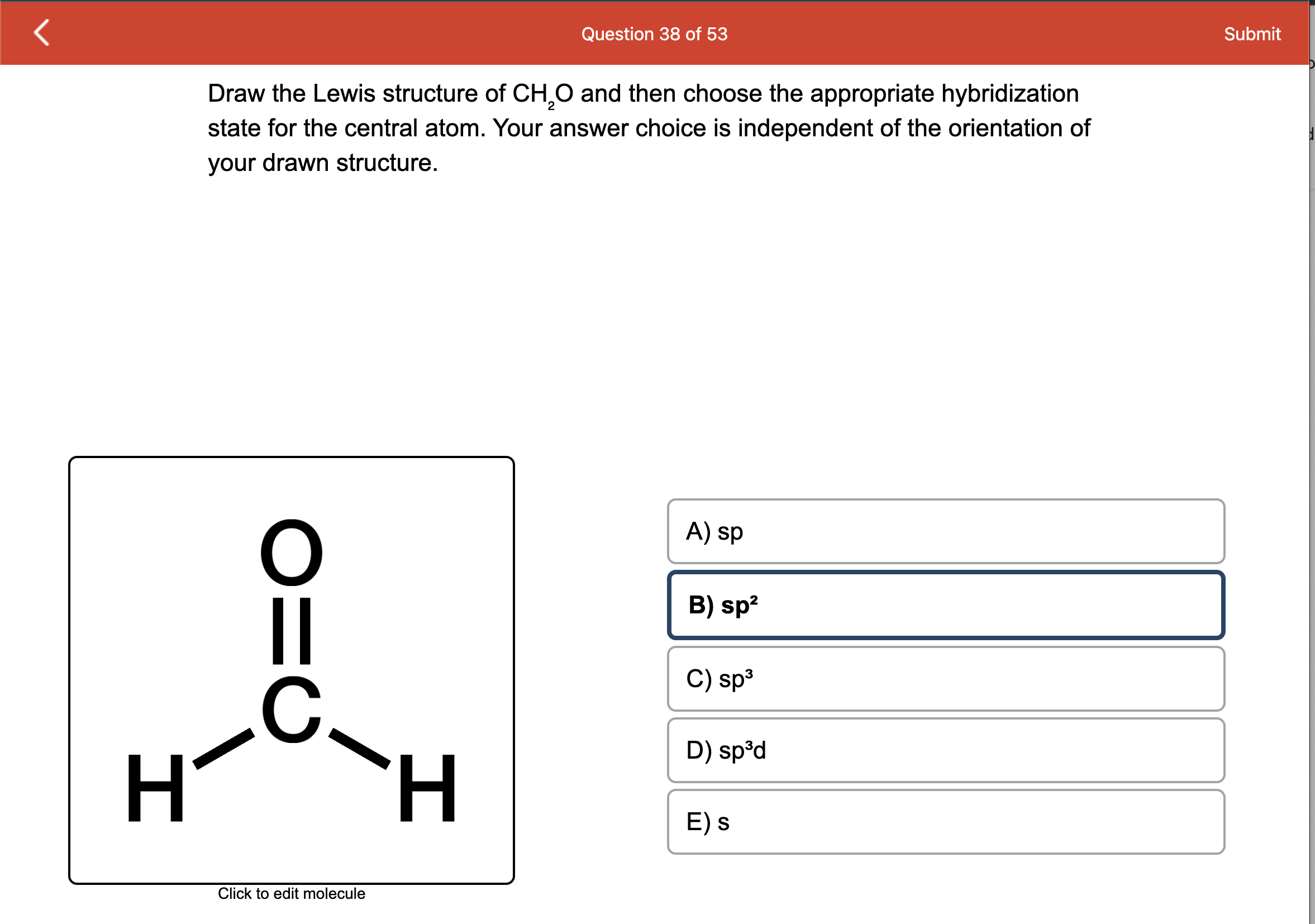
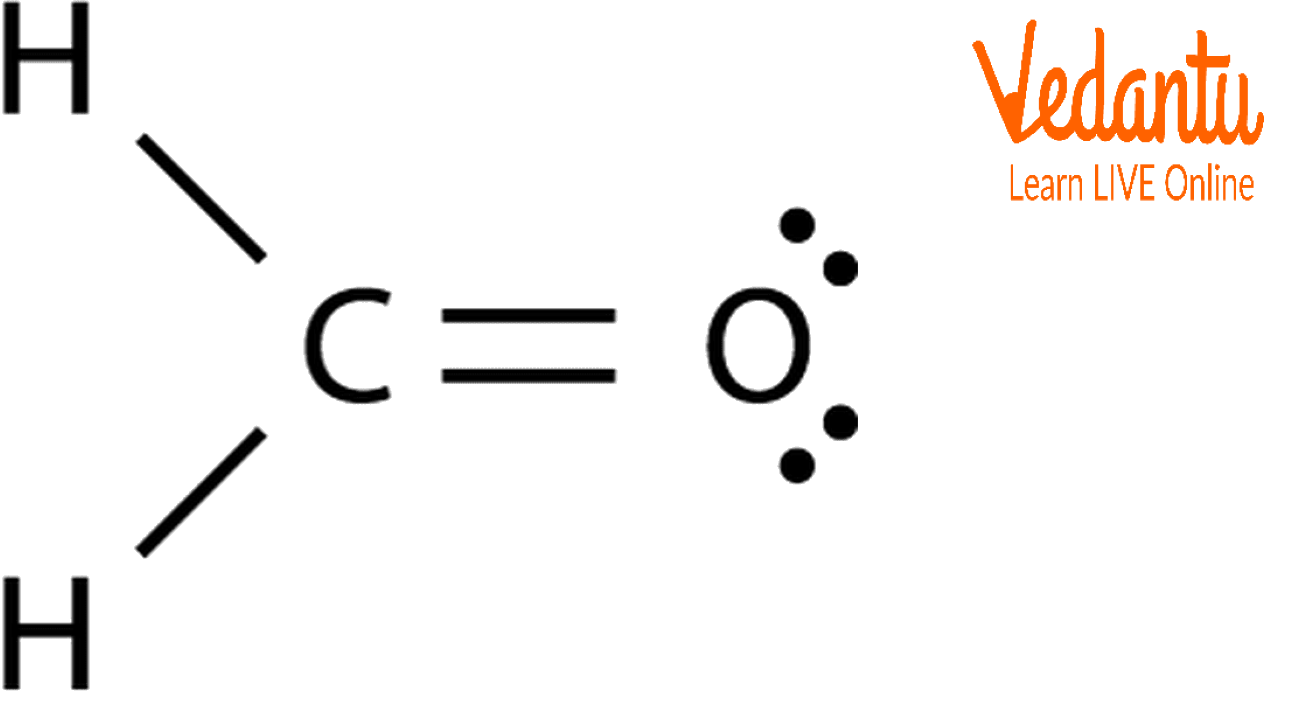CH2O Molecular Geometry,Shape and Bond Angles (Formaldehyde) | Molecular geometry, Molecular, Geometry shape

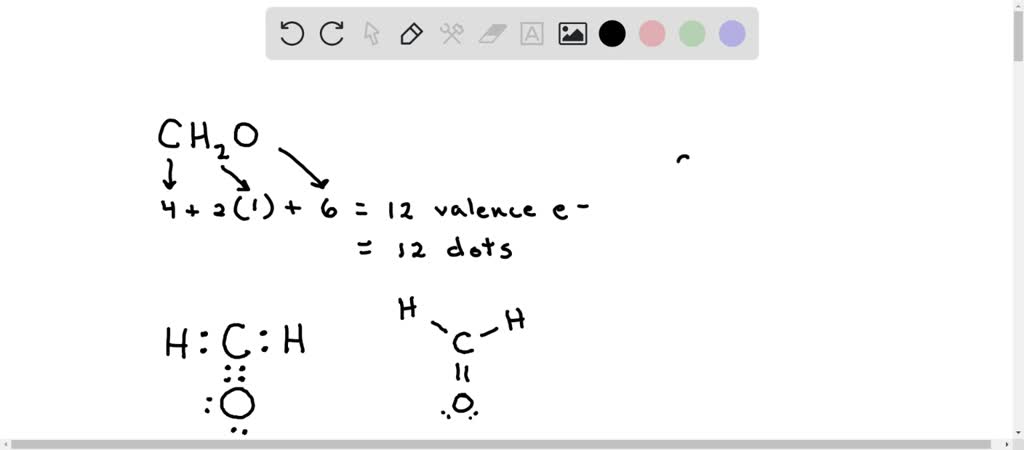
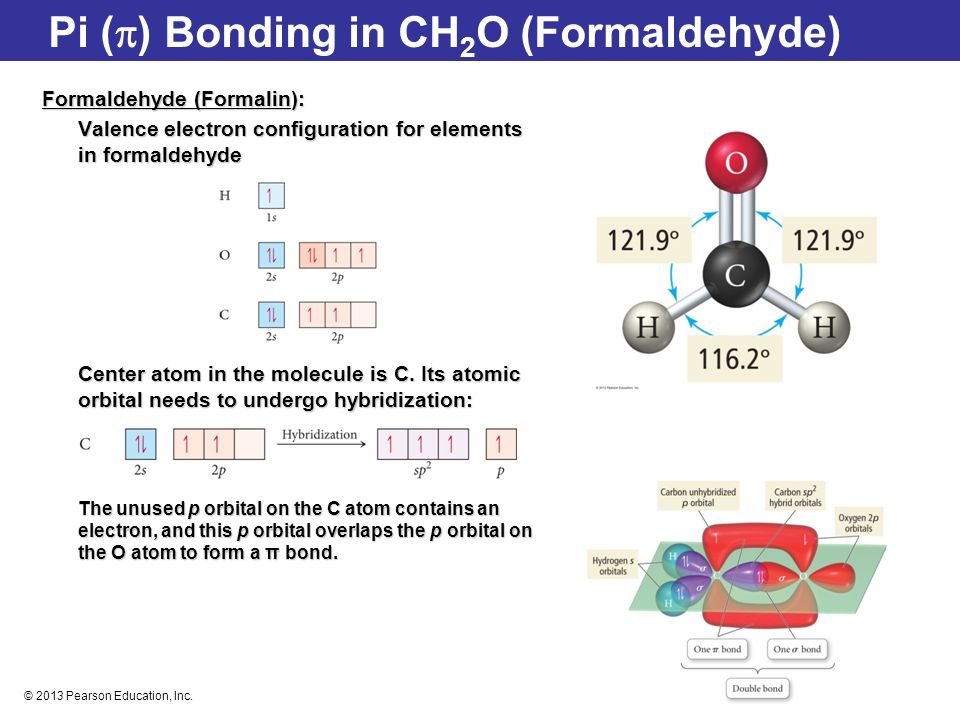
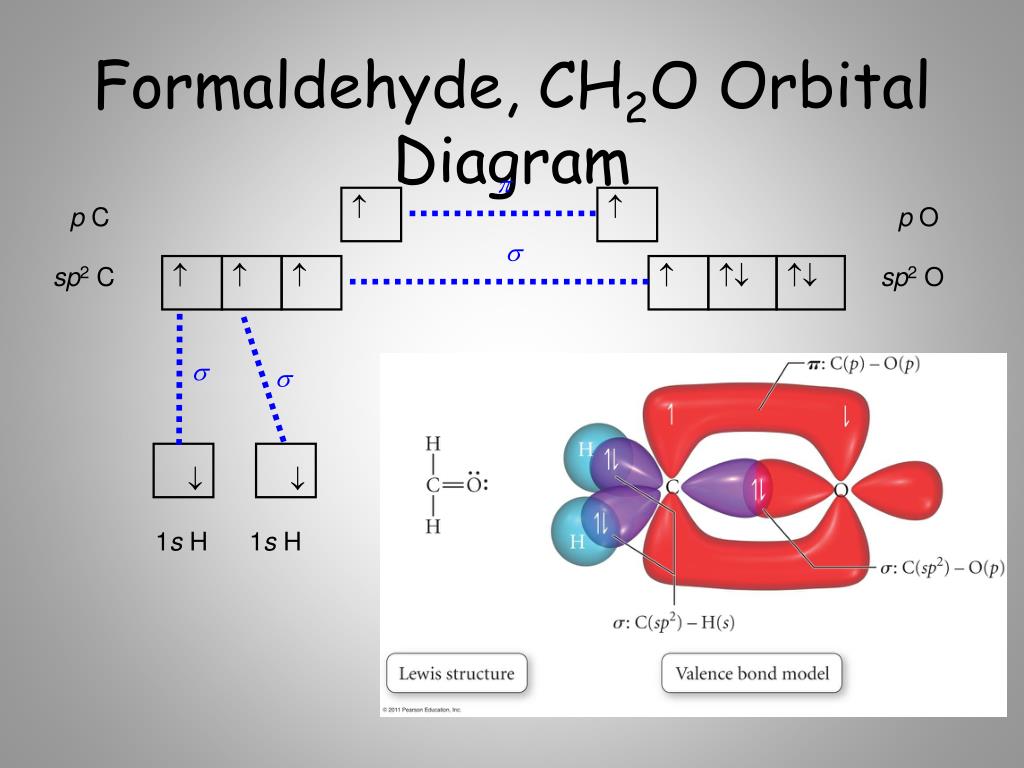
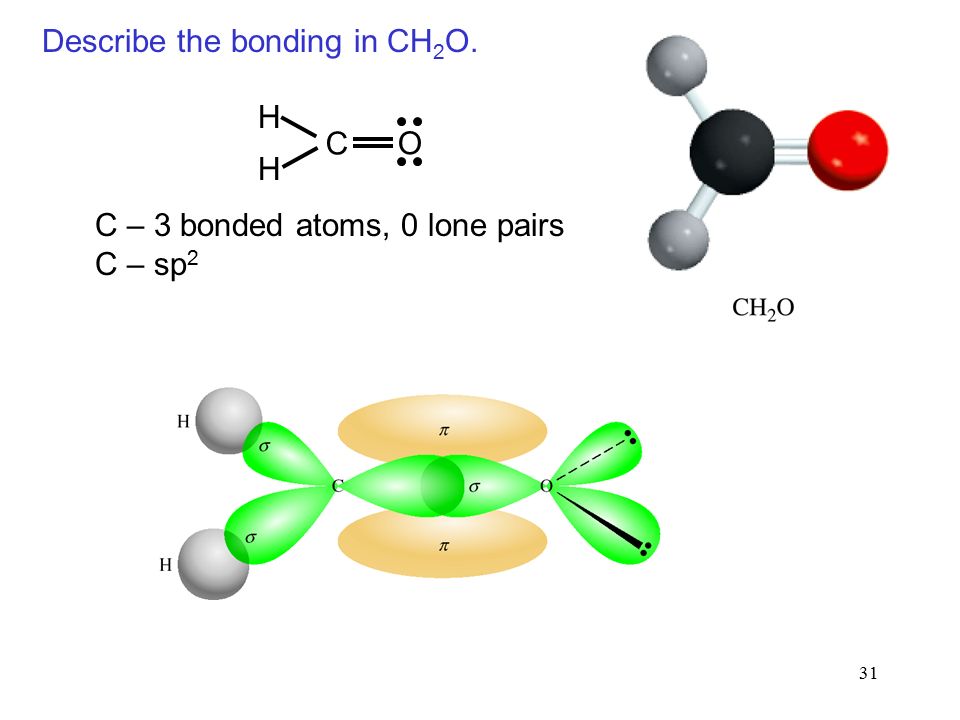
Formaldehyde has the formula H2CO. Describe the bonding between the C and O atoms in VB terms. Formaldehyde absorbs UV light at about 270 nm. The absorption is described as a pi
![SOLVED: Write down the type of hybridization. Example: formaldehyde, CH2O AE = 4 + 2(1) = 12 Lewis Structure NE = 2(2) + 8(2) - 2[4-1] = 4 + 16 - 6 = SOLVED: Write down the type of hybridization. Example: formaldehyde, CH2O AE = 4 + 2(1) = 12 Lewis Structure NE = 2(2) + 8(2) - 2[4-1] = 4 + 16 - 6 =](https://cdn.numerade.com/ask_images/c0886ae8797b4387acbc6bf063645afe.jpg)
SOLVED: Write down the type of hybridization. Example: formaldehyde, CH2O AE = 4 + 2(1) = 12 Lewis Structure NE = 2(2) + 8(2) - 2[4-1] = 4 + 16 - 6 =
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 9 Copyright © The McGraw-Hill Companies, Inc. Permission required. - ppt download